Services offered
Accuprec Research Labs Pvt. Ltd. ® is a service provider for various testing services required for pharmaceuticals, chemicals and phytochemicals as well as herbal formulations.

Biocompatibility Testing of Medical Devices (As per ISO 10993-1:2018)
- In-vitro Cytotoxicity Testing (ISO 10993-5)
- Skin Sensitization Testing (ISO 10993-10)
- Irritation or Intracutaneous Reactivity Test (ISO 10993-23)
Read More

Biological Testing of Raw Material of Plastic, Rubber, Silicone, Polymers, etc.
(As per IP / BP / EP / JP / USP)
- In-vitro Cytotoxicity Testing
- In-vivo Intracutaneous Testing
- In-vivo Acute Systemic Toxicity Testing
Read More
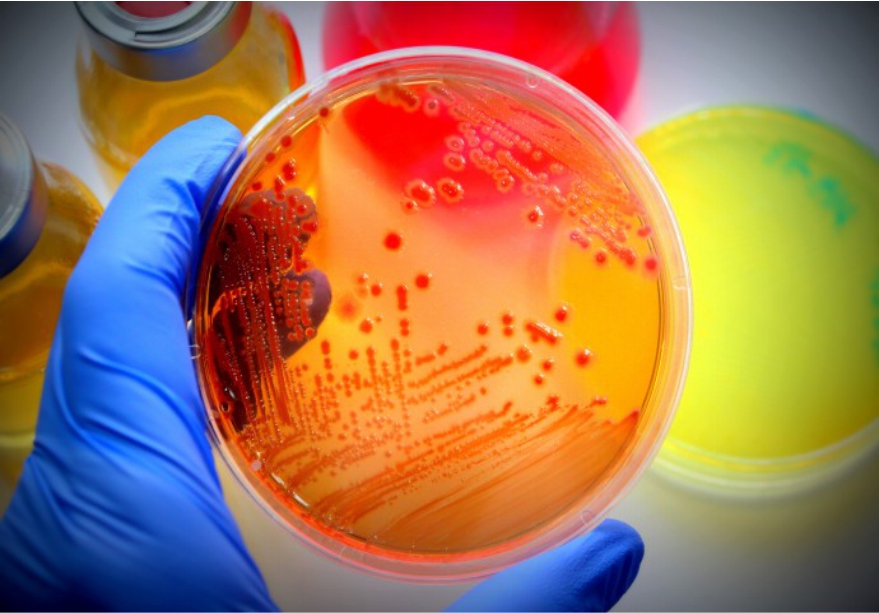
Microbiological Testing Services
- Bioburden Testing & Its Validation (ISO 11737-1)
- Sterility Testing & its Validation (ISO 11737-2)
- Bacterial Endotoxin Testing & its Validation (USP <85> & USP <161>)
Read More
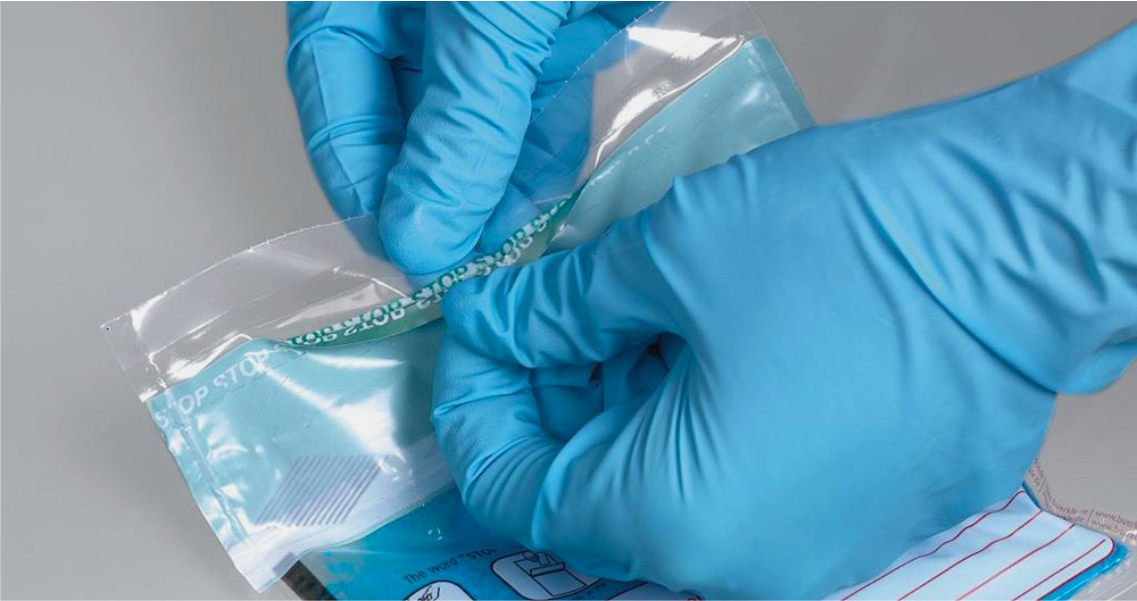
Packaging Testing
- Packaging Integrity Testing & Validation Testing (ISO 11607-1 & ISO 11607-2)
- Performance Testing of Medical Grade Paper & Tyvek Pouch
- Sterilant Penetration Test (ISO 17665-1 & ISO 17665- 2, AAMI/ANSI/ISO 11135-1)
Read More

Stability Testing Services
- All zones Stability Testing available
- Accelerated Stability Study (ASTM F1980, ASTM D7160)
- Real Time Stability Study (ASTM F1980, ASTM D7160)
Read More
Mask, PPE, Gloves & Textile Testing
- Surgical Face Mask (IS 16289, EN 14683 & ASTM F2100)
- N95/FFP Face Mask (ASTM F2100, EN 149 & NIOSH)
- PPE kit (IS 16546 / ISO 16603)
Read More
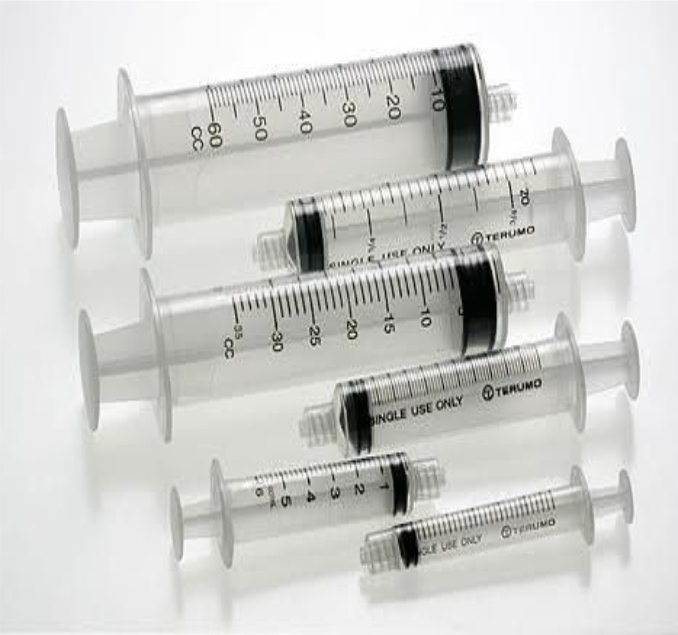
Performance Testing of Medical Devices
- Hypodermic Syringe (ISO 7886-1, ISO 7886-3, ISO 7864, ASTM F3212)
- Sterile single use syringe for Insulin (ISO 8537)
- Stainless Steel needle tubing (ISO 9626, IS 10654)
Read More
Performance Testing of Rapid In-Vitro Diagnostic Kits
- HCG diagnostic kit
- Malarial diagnostic kit
- Blood Glucose monitoring kit
Read More
Research & Development Services
- Development of In-vitro model to prove efficiency of Medical Devices.
- Development of In-vivo model to prove efficiency of Medical Devices
- Development of Chemical sensors for Rapid diagnosis purpose
Read More

Clinical Study
- Clinical Evaluation Report (CER) as per the requirement of EU MDR 2017
- Clinical trial of Medical Device as per ICMR, CDSCO, EU MDR and ICH-GCP guidelines
Read More

Regulatory Dossier Preparation Services
- Preparation of Medical Device Dossier for EU(Europe) market
Read More

Physico-Chemical Testing of Raw Material & Finished Medical Devices
- Chemical testing of Nylon Resin used in Packaging of Food material and in Medical Devices (21 CFR 177.1500)
Read More

IPR Management Services
- Patent Search, Drafting & Filing
- Trademark Search & Filing Services
- Industrial Design Search & Filing Services
Read More

Analytical Testing of Pharmaceuticals, Cosmetics, Medical Devices & Agrochemicals
- Analytical Testing of Pharmaceuticals & Cosmetics
- API characterization and analysis Analytical method development
- Bio-Analytical Method Development and Validation
Read More

Biotechnological Services
- Cell-Line Study for New Molecules
- Determination of Immunoglobulin using HPLC
- ELISA Testing
Read More
Microbiological Services
- Antibiotic Assays
- Assay of Vitamins
- Bio- Burden Testing
Read More

Bio – Compatibility Studies of Medical Devices
(As per ISO 10993, USFDA and EU MDR Guidelines)
- Cytotoxicity Study (In-Vitro)
- Sensitization Study (In-Vitro & In-Vivo)
- Irritation or Intracutaneous Reactivity (In-Vitro & In-Vivo)
Read More
Preclinical & Toxicological Services
(As per OECD, NDCT, ICH, MHRA, USFDA/EPA Guidelines)
- Acute Toxicity Studies (Oral, Dermal, Inhalation and Parenteral)
- Eye/ Skin Irritation Studies
- Skin Sensitization Studies (GPMT/ Buehler)
Read More

Phytochemical & AYUSH Testing Services
(As per AYUSH and other Guideline)
- Description, Identification (Using TLC & HPLC/HPTLC)
- Physico-chemical testing of Raw material and AYUSH formulations
Read More

Food Testing Services
(As per FSSAI, BIS, APEDA and EU Guideline)
- Analysis of Food & Agricultural Products as per FSSAI, BIS, EIC/EIA, APEDA & European (EU) Standards
- Analysis of Residual Pesticides, Drugs and Banned colorants in food products
Read More

Formulation & Development Services
- Controlled release and Sustained Release Formulations
- Excipient Compatibility Selection and Optimization
- Formulation Development For New Chemical Entities (NCE)
Read More
Dyes, Pigment & Plastic Testing Services
- Chemical testing of dyes & pigments of Textile & color Industry
- Heavy metal Analysis
- Particle Size Measurement
Read More
Research & Development Services (DSIR approved R & D centre)
- Design & Development of Patent & Propriety Herbal Formulations.
- Synthesis & process development of Pharmaceutical Herbal
Read More

Stability Testing Services
- Accelerated and Real time stability testing of pharmaceuticals & chemicals as per ICH Guideline (ICH Q2R1)
- All Zones Stability Testing are available including walk in stability chamber
Read More

Clinical Services
- BA/BE Study of Drugs as per the Regulatory guidelines of the Particular country Specified by the Sponsor.
- Clinical site management as per requirement by the sponsor.
- Clinical Trial of Phase II, III and IV for Synthetic Drugs and Herbal Drugs as per schedule Y, ICMR and ICH-GCP guidelines
Read More

Regulatory Dossier Preparation
- Preparation of CTD & e- CTD Dossier for countries like USA, Canada, Europe, Brazil.
- Preparation of Dossier for Allopathic Formulations as well as Herbal Formulations also.
Read More
Accuprec Research Labs Pvt. Ltd. ©
2026












































